Harold Evans
Newly exposed files show how victims were betrayed by political interference in trial – and how the pill has remained on sale
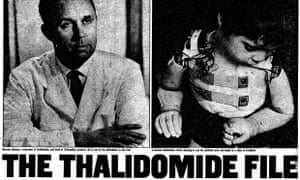
The dark shadow of thalidomide is still with us. The original catastrophe maimed 20,000 babies and killed 80,000: war apart, it remains the greatest manmade global disaster. Now evidence has been uncovered that the pharmaceutical outrage – it is nothing less – was compounded by a judicial scandal that has suppurated all these years.
It is exposed in a large number of documents discovered in the state archives of North Rhine-Westphalia by a researcher for the UK Thalidomide Trust. The papers, which have been examined and authenticated by the international law firm of Ince & Co, speak to political interference that violated the constitutional division of power between the legislative, the executive and the judiciary. And more than half a century since the pill’s threat to an embryo was proven, the company that produced the first disaster has continued to sell the drug in parts of Latin America, on prescription only, where babies continued to be born with malformations similar to the survivors from the 1960s.
The criminal trial of employees of Chemie-Grünenthal, the German company that created and marketed thalidomide, opened in the pretty town of Alsdorf, near Aachen, on 27 May 1968. It promised to be comparable in scale and emotional intensity to Nuremberg. Thousands of deformed babies had died or been allowed to die. Many families with surviving children filed civil suits, but all the victims had to wait years without support because the criminal trial took precedence.
Grünenthal had insisted that it was blameless: the thousands of abnormal births were an act of God. It had the discreet support of the politically well-connected chemical industry, mindful that a conviction would raise insurance premiums. The North Rhine-Westphalia public prosecutors found the company obstructive. They had to seize the most important Grünenthal documents in police raids on its “bunker” and a company lawyer’s house.
It took them six years to examine 5,000 case histories: expectant mothers who had taken thalidomide and given birth to deformed and dead babies, and men and women who had suffered irreversible nerve damage. The bill of indictment they prepared against nine Grünenthal employees ran to 972 pages. In support, they had lined up 351 witnesses, 29 technical experts and 70,000 pages of evidence. There were 400 co-plaintiffs.
Nearly 700 people crowded the biggest space in the region, a “casino” in the premises of a mining company. Every day, the judges – three professional, two lay – lawyers and scientists, press and witnesses, passed by three deformed children nursed by Red Cross sisters while their mothers were inside hoping to learn why they had suffered.
Grünenthal came defiantly to court. Its battery of 40 lawyers, easily outnumbering the prosecution’s, did their best to delay. They several times threatened to walk out. Eighteen of them spent 12 days failing to shake the key testimony of the Hamburg paediatrician Dr Widukind Lenz, who had alerted Grünenthal to the tragedy.
It was reckoned that with court sittings three days a week the trial would take at least three years. It didn’t.
It was shut down on 18 December, 1970. The nine men charged with intent to commit bodily harm and involuntary manslaughter went free. The judges said this was with the explicit approval of the prosecution. They granted Grünenthal immunity from any further criminal proceedings. Silence was imposed on the 2,554 German families who had children with foreshortened limbs or no limbs; many had damaged organs, and some were blind. Their parents then had no alternative but to go along with a miserable compensation scheme contrived by the government and the company. It benefited Grünenthal far more than it did the victims.
What should have happened for justice to prevail was for the government to support the families while the criminal court tracked liability for an enormous crime. That was demanded by the West German Social Democratic party in opposition in 1962, but they forgot about it in government.
Instead, while the witnesses testified and endured cross-examination in noisy, angry scenes in the courthouse, the real action was elsewhere. The large number of private documents newly discovered in German state archives by the researcher for the UK Thalidomide Trust speak to government interference in the judicial proceedings.
On July 21, 1969, the documents show, Grünenthal directors and their lawyers met in secret with the federal health ministry. The principal defendant in the criminal trial had been excused attendance in court for health reasons, but he was there at this and other meetings: Grünenthal’s founder, Hermann Wirtz, a 71-year-old father of five, a member of a devout Catholic family socially prominent as philanthropists in Aachen. No victims or their representatives were present, nor were they advised of the meeting.
A follow-up note to the July meeting records that on 18 September, four federal ministries were involved in discussing an “overall solution”, meaning a high-level political intervention to stop the trial.
The most glaring conflict of interest detailed in the archives is of one Dr Joseph Neuberger (1902-1977). He was a partner in the law firm Neuberger, Pick and Greeven engaged by Chemie-Grünenthal. In November 1966, he personally took over the defence of Wirtz. Three weeks later, on December 1966, he acquired the power to control the prosecution.
How on earth could this happen? There was a political coup that led to an SPD/FDP coalition. Neuberger (SPD) got the job of minister of justice for North Rhine-Westphalia, a post he held from 1966-1972.
Three days before he took office, he wrote to the prosecutors to demand they stop proceedings against his client: “I would be personally obliged for a rapid execution.”
The last thing he did in the afternoon of the day he was sworn in was meet with the prosecution in Aachen. Again, he asked them to stop action against Wirtz. He told them he was resigning as solicitor for Wirtz on becoming minister of justice. But then he discussed the case again and repeated his claims. His personal interest in defending Wirtz and the company persisted, as did his company’s representation.
In February 1970, prosecution officials prepared working papers for and against discontinuing the trial. On 26 January, Grünenthal had offered a glimpse of what it had been brewing in the secret talks. It announced that if the 2,544 families would give up their civil suits for compensation, Grünenthal would contribute 100m Deutsche marks ($27m) to a fund. There was no mention of a deal with the government or ending the trial. The Grünenthal posture was packaged as one of concern for families. The company said it intended to fight the criminal trial and then fight civil actions and appeal. All this would take at least 10 years and meanwhile the children would have no money.
Here, then, was an attempt to lay the ground for closing the criminal trial. The discovered papers suggest the private argument had been going the other way. They include a draft for the prosecution which added up to an argument against closure. The papers are undated but the context suggests February 1970. They considered acquittals were unlikely. Only one argument was advanced in favour of ending the trial. It was that the sooner it was over, the sooner the victims could start civil suits for damages – but that would mean they would receive less compensation than if there had been convictions for negligence.
The papers said discontinuing the trial must meet three conditions. One, discontinuance had to be clearly in the public interest. Two, it had to be shown that the accused had a low degree of personal guilt. The document concluded neither of these two requirements had been met, but even if they had there was an insuperable third: discontinuance could not be considered for any case where “severe bodily harm” was “in the air”. Why then did the prosecutor, reporting to Neuberger, consent to the discontinuance of the criminal trial?
The state prosecutor Josef Havertz said “everything got worse” after Neuberger took over. “I was the only state prosecutor and I was drowning in the files. It wasn’t until the last year I was provided with two young prosecutors as assistants, and they went behind my back and betrayed the victims.”
The betrayal was twofold. The first betrayal was of values. No civilized society can abandon finding out how thousands of babies died, how thousands were deformed, how thousands of adults were inflicted with irreversible nerve damage.
The second betrayal was that the trial was abandoned in circumstances that undermined the parents’ claims for damage, exactly as the ministry briefing had said would happen with premature closure. So not only did the German government of the day secure the release of Wirtz and his associates, but the German taxpayer, not the company, has ended up paying the lion’s share of compensation.
The parents were pressured to end their civil suits. The scheme that emerged from all the secret meetings without parents amounted to just 10% of their claims (an average sum of $22,000 for each child for a lifetime of disability). It stayed low for nearly four decades until, in a dramatic two-step improvement, between 2008 and 2013 the government raised the payments to the most injured to $6,6912 a month. The Grünenthal contribution is now less than 3%.
The UK Thalidomide Trust says it will give the documents it found to the North Rhine-Westphalia’s ministry of health, which has commissioned an investigation into “the Contergan scandal” – the brand name of thalidomide in West Germany. The ministry says the inquiry, to be carried out by Wilhelms University Münster, will focus on the regional state authorities and report in 2015.
The federal ministry of justice refused to comment on the findings in the archives, bringing a brisk rebuke from Dr Martin Johnson for the trust. “We welcome the action of North Rhine-Westphalia but we ask the federal government to answer for its major role in obstructing justice at the time. This is not just about the needs of today’s survivors of a crime, but about justice for the many thousands of children killed by Grünenthal’s drug. The thalidomide disaster remains the worst and biggest crime in postwar Germany. Justice must be done and seen to be done.”
The Wirtz family’s Chemie-Grünenthal, based in the little Rhineland town of Stolberg, was new to pharmaceuticals. Before the war, the family had been in business making soaps, perfumes and cleaning fluids. Hermann Wirtz, who founded Grünenthal in 1946, installed a half-dozen scientists and technicians under the command of 32-year-old Dr Heinrich Mückter (1914-1987).
Wirtz and Mückter had been members of the Nazi party, which was not unusual in postwar Germany. The distinctive feature of Wirtz’s company was the presence of the number of scientists who had experimented in Hitler’s labour camps. The non-executive chairman was Otto Ambros, one of the inventors of the nerve gas sarin, and in charge of the construction of the Auschwitz IG Farben plant. He was sentenced to eight years in prison at the Nuremberg trials, released after four to help the US army chemical corps.
Mückter experimented with live prisoners in labour camps in Poland in his attempt to find an anti-typhus vaccine. Many died. He narrowly escaped arrest by the Polish war crimes prosecutors.
Grünenthal also had Dr Martin Staemmler, one of the top people in the Nazi racial hygiene and eugenics setup. They were not men imbued with exceptional compassion, but Neuberger, who came to the rescue of Grünenthal, could hardly be suspected of Nazi sympathies. He had escaped prewar persecution as a Jew by emigrating to Israel, but returned in time for the German economic miracle. In fact, as relevant to the disaster is the atmosphere in West Germany in those hectic years. It had what a Swedish observer called “an overweening belief in the divine right of commercial success”. There is a Neuberger Medal awarded to non-Jews who helped Jews; Chancellor Merkel was honoured in 2008.
Wirtz and Mückter launched the company’s “wonder drug” for over-the-counter sale in liquid and pill form on 1 October 1957, as a sedative for sleeplessness and remedy for morning sickness in pregnancy. It came to be the second best selling pharmaceutical in Germany, behind only Bayer Aspirin.
Between the launch and the grudging withdrawal on 27 November 1961, Wirtz and Mückter massively promoted thalidomide in 52 countries as “completely non-poisonous … astonishingly safe … fully harmless … completely safe for pregnant women and nursing mothers without any adverse effects on mother and child”. The claim was a fraud. They did no tests for the effect on an embryo. Their stock line later was that nobody in the 50s realised a pharmaceutical could reach the foetus. It was a nonsense statement then, and has become obscene with every repetition.
Round the world, the non-toxic claim of Grünenthal was shattered before the epidemic of malformed births was realised. Adults who took thalidomide as a sedative suffered serious nerve damage. During 1959, Grünenthal received a series of complaints from the German doctors and thalidomide distributors. The management had a standard answer: “First we’ve ever heard of anything like that.”
The first description in medical literature could not be brushed off. In December 1960, the British Medical Journal published a description of four cases of peripheral neuritis traceable to thalidomide. (Distaval in the UK.) Mückter, whose income was tied to sales, already knew of 150 cases, but he told the complaining British distributor to sit back and enjoy the revenues. “More than a million people in West Germany alone take Contergan every day.” Oh, yes, added Mückter, they’d heard of “occasional” reactions – “allergies” most likely – but they vanished after the patient stopped taking the pill. This was another lie, embellished by sales executives who worked hard to suppress bad reports, offered bribes for favourable mentions, and abused troublemakers.
Grünenthal did release some investigative energies. It instructed its private detective, Ernst Jahnke of Essen, to put complaining patients and doctors under surveillance.
To deny, delay and obfuscate was Grünenthal’s routine reaction. It did not act with urgency on abnormal births until doctors in Germany and Australia insisted on the connection. The archived files unearthed by the UK Thalidomide Trust include evidence indicating that Grünenthal kept selling thalidomide longer than was realised at the time of the trial. It took its Contergan brand off the market on November 27, but the documents suggest that Grünenthal had known of the toxic effects for at least six weeks before then – six weeks that imperilled thousands of expectant mothers.
The files recovered include an incriminating letter by Dr Günter von Waldeyer-Hartz. He was a chemistry graduate who had been in a training unit for scientific sales in October 1961, six weeks before the drug was withdrawn. On January 5, 1969, realising his evidence might not surface in the trial, he sent a letter by registered post to the minister responsible for the liaison between state and federal, one Dr Posser: “We were shown a packaging unit for Contergan-Thalidomide with the sticker NOT FOR PREGNANT WOMEN. The management knew about the intra-uterine effect of the preparation at the latest by mid-October. The decision to carry on selling the preparation was without any doubt motivated by profit-making and criminal in my view.”
He named four witnesses. A note by prosecutor Havertz (26 January 1969) says he recalled sending the great criminal chamber testimony from Dr Waldeyer-Hartz in “the spring or summer of 1968”. It was never found.
The judges who acquiesced in the closure sanctioned by Neuberger did not convict and they did not acquit, but in the final remarks, they said the substantive charges had all been legally proven. The nerve damage would have been easy to foresee. It was as “a well-known fact” that a great number of chemical substances were suspected of being harmful to the foetus. The company had been “negligent, misleading, inexcusable, unlawful, very inadequate by the standards of the day … obstructive … unethical …”
Yet, inexplicably, the judges then granted immunity. Extenuating circumstances? They expressed sympathy for the personal suffering of the accused . The unethical practices from excessive competition were “regrettably” widespread due to a lack of federal regulation. The company had voluntarily “made considerable contributions from their private fortunes for the benefit of destitute victims”.
Grünenthal has been unrelenting in exploiting its get-out-of-jail-free card. It has a regular excuse for washing its hands of the tragedy it created. It insists it has no legal liability (thanks to the aborted trial). It suggests it was just unlucky because it followed “the standards prevailing in the pharmaceutical industry at that time”. This assertion merely lengthens Pinocchio’s nose since whatever they thought erroneously in 1958 they have kept saying since in the face of repeated scientific testimony of its falsity.
Reproductive studies had been routine at pharmaceutical companies since the mid 1940s. As the German judges said in their final remarks in 1970, it was widely recognised at the time that a drug could indeed reach the foetus. The tranquilisers in direct competition with thalidomide were all tested for teratogenic effects and the results published. “The overall behaviour of the company did not correspond with the standards required of a serious and conscientious producer of pharmaceuticals,” the judges said.
And yet, Sweden and Switzerland apart, government and law mostly abandoned the children to decades of David and Goliath legal suits; parents with limited resources but limitless new obligations of care versus powerful and immortal corporations.
A hundred victims in Australia and New Zealand were left to struggle unaided for 40 years until Ken Youdale of the Australian Thalidomide Trust and lawyer Peter Gordon won a class action suit against Grünenthal in December 2013. The responsive British multinational Diageo, which inherited thalidomide as a licensee, voluntarily put $88m into a fund for 100 victims. Grünenthal refused to contribute. A hundred Canadian victims today live miserable lives because their disabilities have aged them beyond their years and outrun the small provisions from Grünenthal’s distributor Richardson-Merrell and the government. It is excruciating to see the fragment of a video of a Canadian woman who can take a bath only when there is someone around who can try to get her into a sling so she can be winched in and out of the water.
In Spain, where thalidomide was available until 1965, Grünenthal keeps fighting a case mounted for 180 people by the Spanish Association of Thalidomide Victims (Avite). Victims arrived at the courtroom in their wheelchairs on 21 November 2013 to cheer Grünenthal’s conviction. It was ordered to compensate each one of the plaintiffs according to the severity of their injuries. But then Grünenthal got the judgment reversed in November 2014 on the grounds they had sued too late. Now Avite has to raise more money to take the case to Spain’s highest court, while one of Grünenthal’s undoubted victims, Juan Carlos Vélez, 44, sits on the pavement in a smart part of Madrid with a hat upturned for coins. He can’t hold out a hand because he hasn’t any.
Grünenthal seems to believe its moral obligation has been discharged by its creation of a foundation for palliative care in Aachen. That is no solace for the victims worldwide. The 300 Spanish members of Avite are upset that Pope Francis I has honoured Michael Wirtz, the son of Hermann, the founder of Grünenthal. Michael Wirtz is no longer chairman, but he is seen as a symbol of its obduracy. No mention was made of thalidomide in a ceremony in Aachen earlier this year. Bishop Henry Mussinghoff conferred on him the Order of St Sylvester, “one of the highest honours which the pope gives to lay persons for services to the Roman Catholic church”. Avite will petition the pope to rescind the award. Grünenthal’s possible impact in the pope’s home nation of Argentina seems to have escaped attention.
Health researchers believe that many cases of second and third generation victims in Argentina are because some of the companies did not impose stricter restrictions on its sale. The records of the Nacional de Meckcamen show Grünenthal selling thalidomide (No 2066) at least until about 2000 under the trade name Talidomida Cassara. Eight different manufacturers made thalidomide. Six of them had the sales restriction, making it exclusively for use in hospitals. Two, Grünenthal’s Cassara and one other, however, required only a prescription, meaning anyone with a prescription could get it by walking into any pharmacy. The researchers say that this meant that, although the products would have carried inserts in the packaging warning of the risks, people could take pills home and share them without control. Between 1997 and 2005, only one thalidomide baby was born in Brazil after use was restricted to hospitals. Five were born between 2005 and 2008 when this restriction was watered down.
Nobody quite understands how thalidomide works. It has been found to prolong the lives of patients with multiple myeloma and to ease the symptoms of leprosy. The drug is out of patent. Millions of pills are being produced by companies and black market operators with an eye on its potential more than the risks of it being ingested by expectant mothers. The paediatricians in the hospital-based Eclamc (The Latin American Collaborative Study of Congenital Malformations) know of a hundred malformed babies but suspect there are “vast number of innocent victims” not yet known since, the group covers less than 1% of all births in Latin America: “The incitements for a new disaster are alarming”.
Experts on thalidomide meeting in October at the World Health Organisation in Geneva called for enhanced studies of the epidemiology. These redemptive efforts of science may be considered rather more worthy of applause than the pope’s unfortunate embrace of a rich company that escaped accountability in the criminal court, and still refuses to accept responsibility for inflicting pain and suffering on innocents worldwide.
Additional reporting by Madeline Chambers in Reuters Berlin bureau and Tobias Arndt.
Sir Harold Evans is Reuters’ editor at large and co-author with Phillip Knightley, Elaine Potter and Marjorie Wallace of Suffer The Children, The Story of Thalidomide (1979) by The Sunday Times Insight Team.
Grünenthal’s statement to the Guardian
“First of all, we would like to emphasise that Grünenthal sincerely regrets the thalidomide tragedy, which will always be part of the company’s history. Grünenthal cares about those affected by thalidomide and is involved in an active dialogue with those affected in many countries – amongst others in the UK.
“In 1971, the German parliament passed a law for the establishment of the so-called Contergan Foundation to provide monetary relief and life-long pensions to those affected by thalidomide. Grünenthal voluntarily contributed more than €100m for the support of those affected in Germany and other countries through the Contergan Foundation.
“Additionally and in case of a special personal emergency all individuals recognised to have been affected by a thalidomide containing product of Grünenthal or one of its distributors can apply for more support from the company-owned Grünenthal Foundation. Those from the UK and Spain can also apply.
“As regards the litigation in Spain, 160 claims in Spain were dismissed in the first instance; the remaining claims of approx. 20 people have now been rejected by the court of appeal. The claims in Spain did not distinguish between thalidomide products marketed by Grünenthal and those marketed by other companies with no links to Grünenthal. There are established government schemes in Germany for financial support of Spanish individuals affected by a thalidomide containing product from Grünenthal or its distributors. There are individuals who currently benefit from these payments.
“In April 1970, Grünenthal and lawyers representing the affected children and families in Germany entered an out-of-court settlement under which Grünenthal committed to a pay compensation of 100m Deutsche marks to the families. The government and the prosecution were not parties to that settlement nor was it a condition precedent for payment of the settlement amount that the criminal trial pending against officers and employees of the company was discontinued. In fact, the trial continued after the settlement. Only in December 1970, after several years of criminal investigations and more than 280 days in court, did the criminal court of Aachen decide to discontinue proceedings. In accordance with applicable criminal laws, the discontinuation of the proceedings was a decision made by the court on its own assessment and sole responsibility and not a decision of the prosecution.
“The introduction of thalidomide took place more than 50 years ago. Grünenthal believes that its conduct was consistent with the state of scientific knowledge and the prevailing standards for the development and testing of the pharmaceutical industry at that time. Grünenthal believes that medical concern that thalidomide could be responsible for malformations was raised with it for the first time in November 1961 by German physician Dr Widukind Lenz, and then, a few days later, when the UK-based licensee Distillers informed Grünenthal that an Australian physician, Dr William McBride, had raised a similar suspicion. Eleven days after being contacted by Dr Lenz, Grünenthal took steps to withdraw thalidomide from the market.”
• This article was amended on 27 November 2014 to add Marjorie Wallace’s name to the co-authors of Suffer the Children.


No comments:
Post a Comment